ASA Biopharmaceutical report, Fall 2022
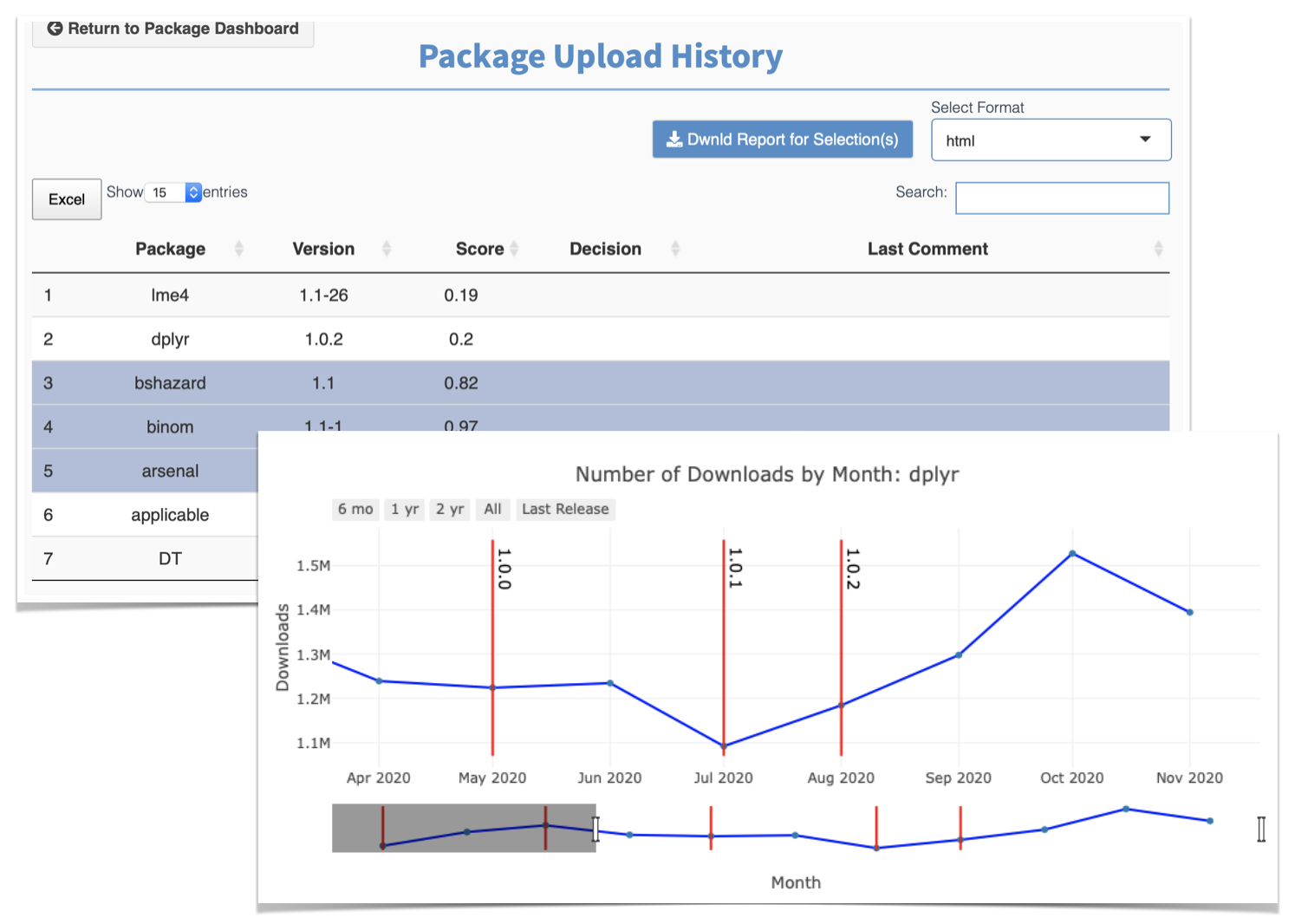
I’m pleased to share that the R Validation Hub’s efforts have been recognised in the ASA Biopharmaceutical report, Fall 2022. Within the edition you can find our paper, Risk Assessment of R Packages: Learnings and Reflections. This paper reflects on our white paper; provides an overview of our {riskmetric} package and Risk Assessment application; and summarises our 2022 case studies (which you can now find on our Case Studies page).